- LATEST
- WEBSTORY
- TRENDING
INDIA
All you need to know about 3 dose Zydus Cadila's ZyCoV-D vaccine to be administered to children
Zydus Cadila developed ZyCoV-D vaccine in partnership with the Department of Biotechnology. India becomes the first country to develop a DNA vaccine.
DNA Web Team | Aug 21, 2021, 02:57 PM IST
1.ZyCoV-D vaccine is needle-free

The ZyCoV-D vaccine is needle-free and is to be administered intradermally using PharmaJet, a needle-free applicator that ensures painless vaccination. This will help in administering the vaccine to kids. This vaccine will be given in three doses - the second dose administered on day 28 from the first dose and the third ones on day 56 from the first dose.
(Image Source: Twitter)
2.ZyCoV-D is the first Plasmid DNA vaccine
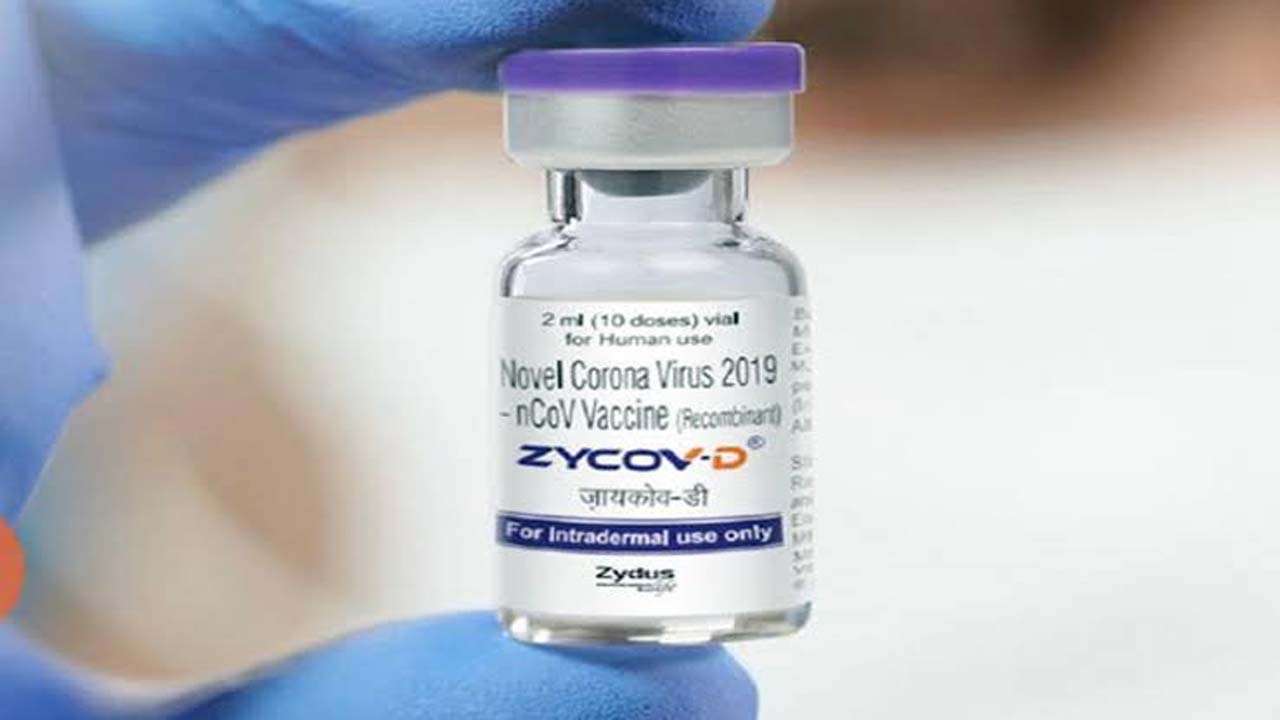
As per experts, the ZyCoV-D vaccine uses a section of genetic material from the COVID-19 virus that gives instructions as either DNA or RNA to make the specific protein that the human immune system recognises and responds to.
(Image Source: Twitter)
3.ZyCoV-D can quickly deal with mutations in virus

The ZyCoV-D doesn't have any problem associated with vector-based immunity because it is a Plasmid DNA vaccine. The Plasmid DNA platform also allows generating new constructs quickly to deal with mutations in the virus.
(Image Source: ANI Digital)
4.ZyCoV-D vaccine is stored at 2-8 degrees Celsius
Unlike Pfizer and Moderna vaccines that need to be stored at very cold temperatures, the ZyCoV-D vaccine is stored at 2-8 degrees Celsius and has shown good stability at temperatures of 25 degrees Celsius for at least three months.
(Image Source: Twitter)
TRENDING NOW
5.ZyCoV-D vaccine effective against Delta variant
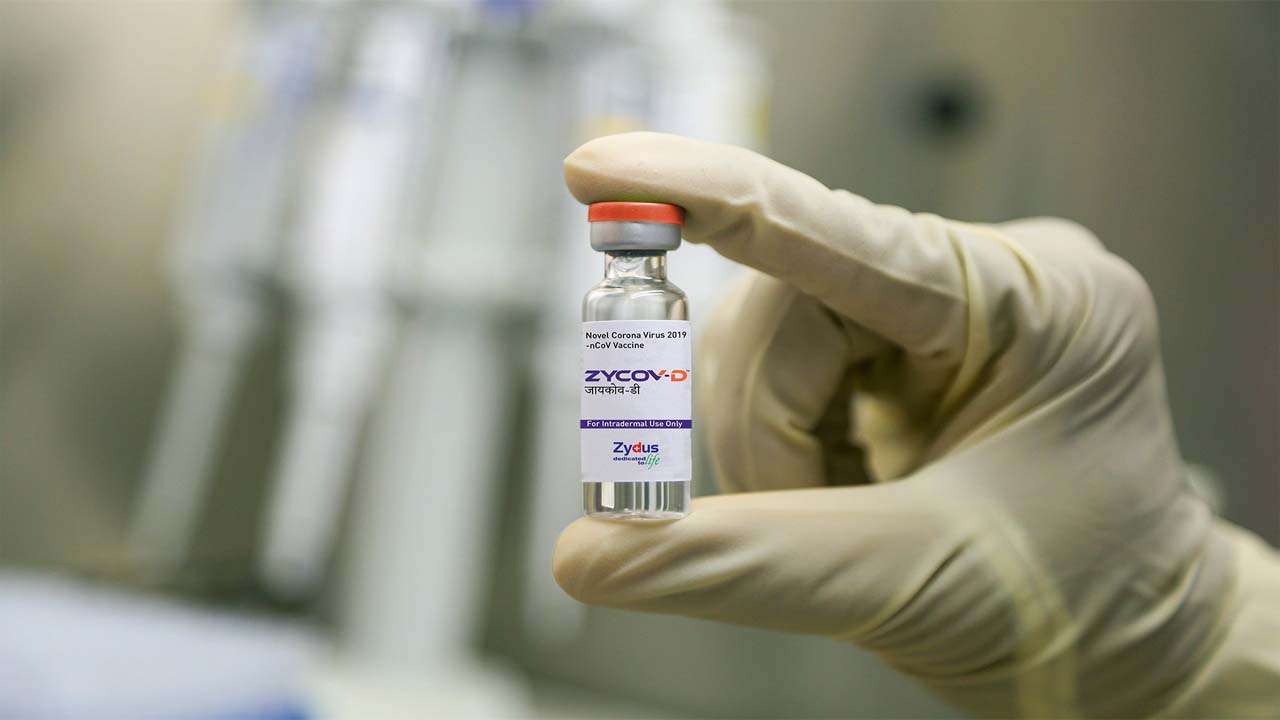
The company has claimed that the ZyCoV-D vaccine is effective against the new COVID-19 variants like the deadly Delta variant. The company plans to manufacture between 100 million and 120 million doses annually and has begun stockpiling them.
(Image Source: Twitter@IndianEmbassyTR)






)

)
)
)
)






























































